Becoming a blood donor is one of the most altruistic acts a person can perform. A study supported by the Association for the Advancement of Blood & Biotherapies’ (AABB) National Blood Foundation showed that 75% of blood donors surveyed said they gave blood to help others, and they were gratified by supporting their community.
In the United States, over 6 million people donate approximately 13.6 million units of blood annually to blood collection centers. This means 4.5 million people can receive life-saving blood each year at transfusion hospitals. Unfortunately, only 20% of blood donations in the U.S. come from people belonging to underrepresented racial/ethnic groups. While this is a 6% increase since 2017, the U.S. population is becoming increasingly diverse. This necessitates an equally diverse blood donor population to ensure compatible blood for treatment is available.
As the demographics of blood donors and those receiving blood are evolving, further understanding of the groups from whom blood is being derived, and those requiring blood transfusions, is needed. Blood donation and blood transfusion clinical trials are essential in elucidating new treatment strategies for which transfusions may be necessary (e.g., patients receiving chemotherapy for cancer, bleeding in the setting of trauma, and anemia). Additionally, some of these conditions that rely on transfusion for treatment disproportionately affect individuals belonging to select groups. Understanding the demographics of individuals enrolled in these clinical trials will instruct future trial practices and ensure findings from these trials are applicable to the overall U.S. population.
There is little information published on the demographics of healthy donors and participants recruited into blood donation and blood transfusion clinical trials. Therefore, in our recent study we investigated the demographic trends of adult and pediatric participants recruited into various interventional U.S. blood donation and blood transfusion clinical trials using publicly sourced data from the largest clinical trial database in the U.S., Clinicaltrials.gov.
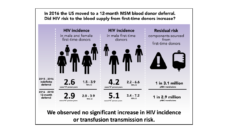
We found gaps in the enrollment of various groups in blood donation and blood transfusion clinical trials. The data also revealed underrepresentation of females and select racial groups in blood donation trials. There was underrepresentation of Asian, Native Hawaiian or Other Pacific Islander, and American Indian or Alaska Native adults and adolescent groups in blood transfusion trials. We also identified minimal participation of older adults (ages 65+) in these trials.
Finally, we highlighted that various racial and ethnic groups were underrepresented in blood transfusion trials dedicated to evaluating transfusion-related interventions for conditions including cardiac disease, anemia, and sickle cell disease.
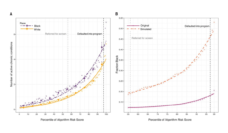
A secondary finding in our study showed that many trial researchers failed to document participant demographics on race and ethnicity compared to sex or age in the Clinicaltrials.gov repository. This both violates federal guidance for reporting all demographic data, and hinders researchers ability to understand the demographics of trial participants. The Clinicaltrials.gov database represents a crucial archive of data. However, even with improved reporting of trial demographic data in recent years, persistent gaps in records of trial enrollees could have an impact on the generalizability of the information needed to instruct future inclusive trial enrollment. We argue that all clinical trials regarding blood should be registered and complete information documented.
In consideration of our findings, we believe various factors may be influencing representation of certain groups in blood donation and transfusion trials. These include unconscious bias by trial practitioners and trial selection criteria and design. We also believe access to multilingual language material and limited accessibility to trial sites may be important factors, particularly as our study found that trial sites were skewed away from regions across the country with larger underrepresented racial/ethnic groups.
To improve trial participation of these groups, we suggest strategies to diversify trial personnel and the fostering of community-based relationships to further engage individuals. Recruitment of diverse groups has also been shown to work when there is provision of transport to trial sites and alternative scheduling outside of business hours.
While our study indicates there is progress in attempts to diversify blood donation and blood transfusion clinical trials, we also believe our findings demonstrate that ongoing disparities in the representation of various groups in these clinical trials are additional barriers to equitable health.
Photo via Getty Images