The Ted Talk crowd is laughing. Kathryn Schultz describes a cartoon: the one with the coyote who endlessly chases the roadrunner. She recalls the episode when the coyote chases the roadrunner off a cliff. The roadrunner is fine because it can fly. For a moment, the coyote is fine too — until he looks down and realizes that he’s in mid-air, that the solid ground he stood on is no longer there. Kathryn Schultz’s Ted Talk isn’t about slapstick cartoons, but about “being wrong.”
In the health world, we rely on science as being “right.” Clinical trials, done strictly, are the scientific gold standard for comparing new treatments, designed to limit bias and include diverse participants. But sometimes certain populations aren’t represented adequately in trials.
How trials measure race can be significantly improved. For example, “Asian” is commonly used as a race category, yet people from East Asia and South Asia may have vastly different cultures, economic status, and health outcomes.
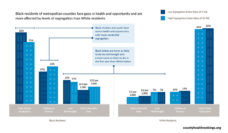
A recent study led by Christopher Aldrighetti considered if certain racial groups were underrepresented in oncology clinical trials. The research team looked at clinical trials of breast, prostate, lung, and colorectal cancer patients. The team found that Black and Latinx people were vastly underrepresented. In contrast, White participants were overrepresented.
This finding was not completely new. In 2019, the Genome-Wide Association Study found that 96% of participants in cancer studies have been White. Over the past 14 years, far fewer Black and Hispanic women have participated in cancer trials compared to White women. Shockingly, 53% of the cancer studies in Aldrighetti’s review didn’t include any information on participant race and ethnicity.
If most of what we know about health and disease is based on people with European ancestry, clinical research may continue to miss some manifestations of cancer in other racial groups.
Modern cancer treatment can be personalized by curating treatments based on a person’s genetics. But if the basis of personalized treatment comes from studies that don’t include a meaningful proportion of one or another racial group, can its findings truly be “personalized”? Underrepresentation of minority groups has been noted in clinical trials for HIV, multiple myeloma, and Alzheimer’s.
Another possible explanation for these disparities is that the researcher population isn’t diverse. A study led by Kenneth Getz found that physicians of color who performed clinical research were greatly underrepresented. They also found that the race of the physician can influence the race of the volunteer participant.
If most of what we know about health and disease is based on people with European ancestry, clinical research may continue to miss some manifestations of cancer in other racial groups. New efforts, like the All of Us Research Program by the National Institutes of Health, are cultivating robust databases based on diverse populations.
Evidence from clinical trials may not be “wrong,” but biases that influence their design create limitations in their interpretation. Policies that regulate clinical trials may limit such disparities. Like the roadrunner, researchers can run off cliffs chasing scientific advancement, under the assumption that the science they follow is fact. But the awareness of researchers who look down and realize that our science may not always be solid is a step towards racial equity.
Photo via Getty Images