The discovery of penicillin is considered one of the great achievements in medicine. Penicillin was the primary contributor to the sharp increase in life expectancy in the 1900s. Without antibiotics, chemotherapy, organ transplants, and joint replacements would not be possible. However, antibiotics are the only class of drugs to lose efficacy with wide-spread use.
In harmful bacteria, antimicrobial resistance genes are the result of overuse and improper use of antibiotics. If antibiotics are taken too often, or if a course of treatment is not properly completed, bacteria can evolve to the point of not responding to a particular type of antibiotic, rendering that medication ineffective. Once this happens, we may have fewer treatment options for the infection, increasing mortality.
Take staphylococcal (aka “staph”) infections for example. A staph infection on the skin in is highly contagious. With effective antibiotics, most staph infections can resolve in a week. However, patients infected with MRSA, a strain of staph resistant to common antibiotics, are 64% more likely to die than patients with treatable infections.
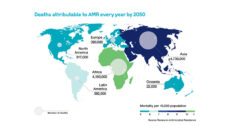
In 2019, 2 million people in the US were diagnosed with antimicrobial resistant (AMR) infections and over 35,000 died. By 2050, AMR is predicted to become the largest killer worldwide.
Laissez-faire medical prescribing, animal feed reinforced with antibiotics to prevent infections and promote growth in feedlots, and the presence of antibiotics in waste water from hospitals and manufacturing plants all contribute to the environmental load of AMR. A recent study reported that 72% of COVID patients received antibiotics even when not clinically necessary. In some countries, 80% of medically necessary antibiotic consumption is in the animal sector.
Drug development is not keeping pace with resistance.
Dr. Helen Boucher recently published a paper highlighting progress in fighting antimicrobial resistance. She highlighted two potential solutions: new drug development and reducing overuse.
Drug development is not keeping pace with resistance. Throughout the 1900s, new types of antibiotics were continuously produced. However, a new class of antibiotics for harmful bacteria has not been approved since 1962. To combat research and development slow-downs, national governments have created incentives to encourage innovation. The 10-20 Initiative, formed in 2010, called for the development of 10 new antibiotics by 2020. Fourteen new products have been approved, but none a novel class of medications.
CARB-X, a non-profit organization based at Boston University, funds and supports new drug development. In five years, CARB-X has funded 92 projects with a handful now in early-stage clinical trials. It is too early to tell how many will be successful.
Boucher’s second point emphasized the importance of antibiotic stewardship in conjunction with drug innovation. Stewardship combines using antibiotics responsibly while maintaining access for patients in need. Proposed legislation can change insurance reimbursement structures so that new antibiotics can reach patients with AMR infections and stabilize the market for drug producers. At the same time, hospitals will need to implement stewardship programs to enforce the proper and responsible use of antibiotics. Oversight of the use of antibiotics in other industries, especially animal populations, also requires stewardship policies.
The global community has recognized AMR as a significant and pressing threat. The causes of AMR are known, the solutions will require consistent attention and resources.
Photo via Getty Images